

Information from the device can also be used to help determine patterns in glucose levels and make long-term adjustments to diabetes treatment plans to keep blood glucose levels in a safe range. PDF The purpose of the study was to evaluate the performance and usability of the FreeStyle() Libre Flash glucose monitoring system (Abbott Diabetes.
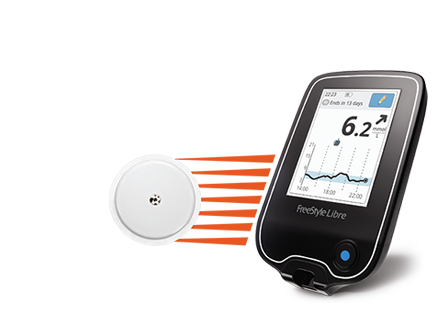
What will it accomplish? People with diabetes can use the information from this device to make diabetes treatment decisions (for example, whether to administer insulin or carbohydrates). The System is intended for single patient use and requires a prescription. sensor requires calibration, make sure to do this when your blood glucose. Food and Drug Administration today approved the FreeStyle Libre Flash Glucose Monitoring System, the first continuous glucose monitoring system that can be used by adult patients to make. Interpretation of the FreeStyle Libre readings should be based on the glucose trends and several sequential readings over time. The sensor in your continuous glucose or flash monitor is taking readings from.
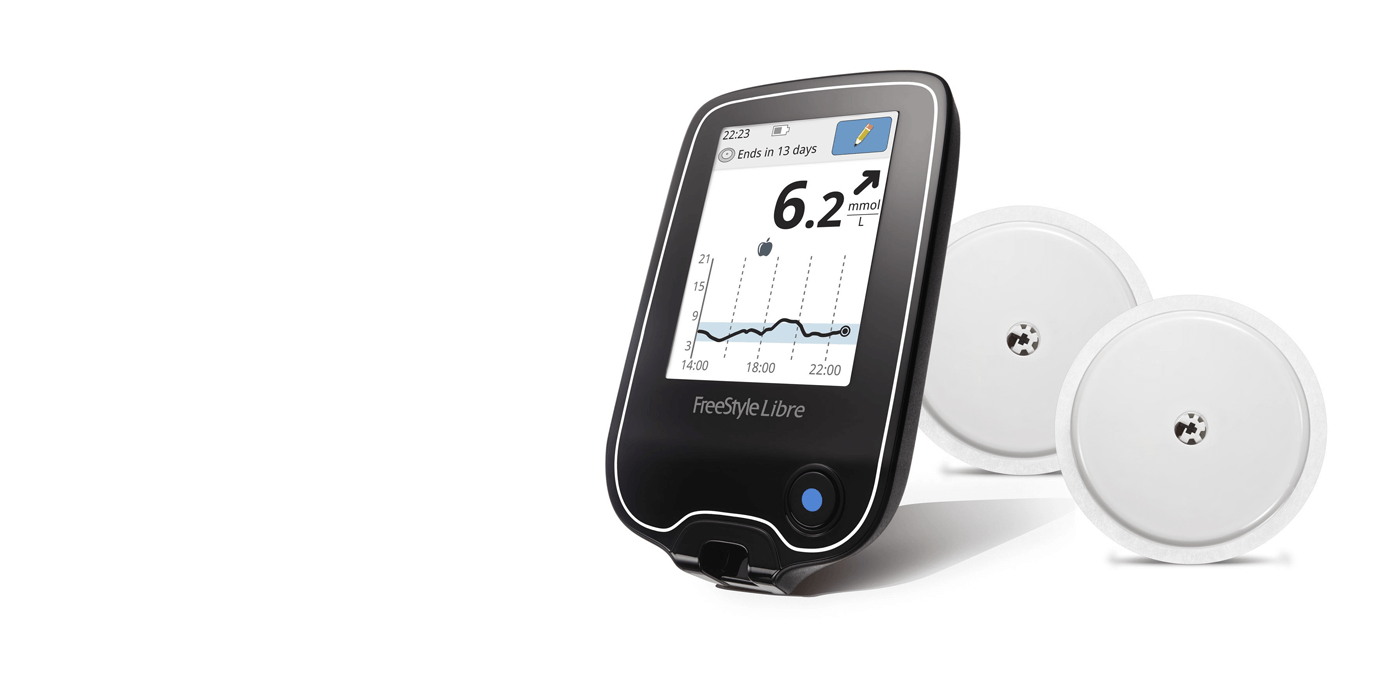
The FreeStyle Libre detects trends and tracks patterns aiding in the detection of episodes of hyperglycemia (too high glucose) and hypoglycemia (too low glucose), to help patients make acute and long-term therapy adjustments. It is designed to replace blood glucose testing with a fingerstick for diabetes treatment decisions. Libre FGM system acceptability at 3- and 6-months, and all participants will answer acceptability. The FreeStyle Libre Flash Glucose Monitoring System is indicated for the management of diabetes in persons age 18 and older. Adolescents in the FGM group will evaluate the FreeStyle.


 0 kommentar(er)
0 kommentar(er)
